For investors and media only
Issued: London, UK
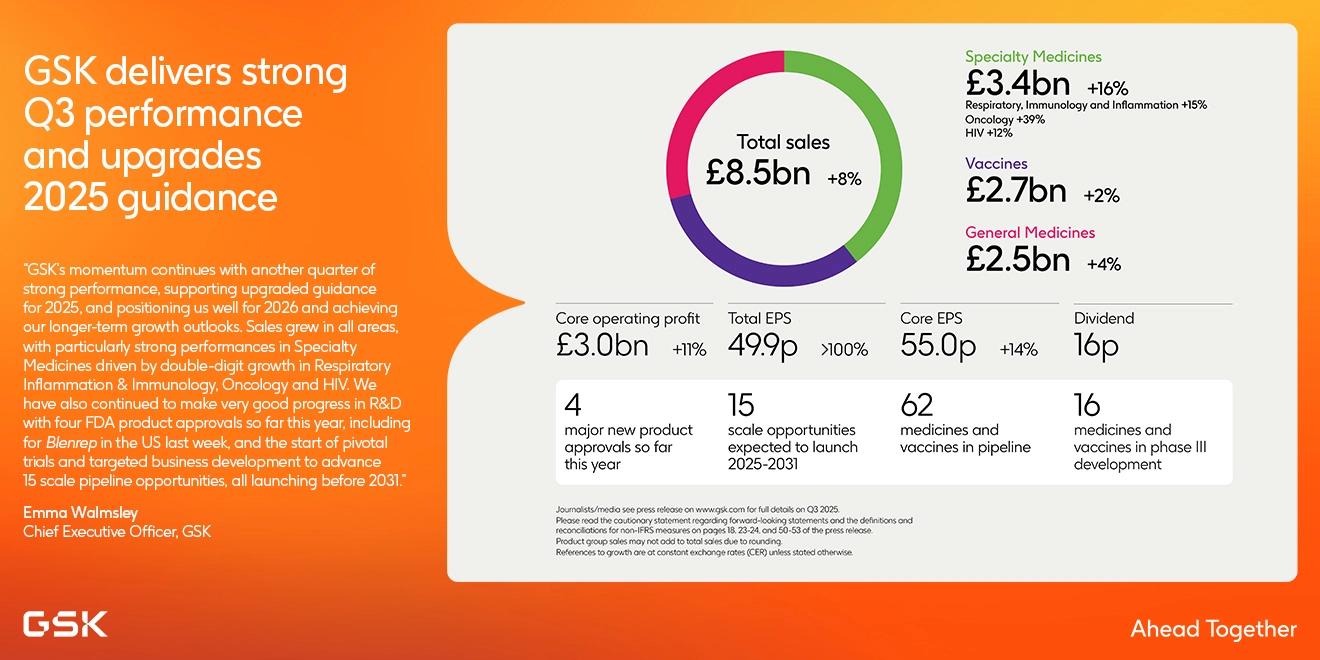
GSK delivers strong Q3 performance and upgrades 2025 guidance
Specialty Medicines, Vaccines and General Medicines drive sales, profit and earnings growth
- Total Q3 2025 sales £8.5 billion +7% AER; +8% CER
- Specialty Medicines sales £3.4 billion (+16%); Respiratory, Immunology & Inflammation £1.0 billion (+15%); Oncology £0.5 billion (+39%); HIV sales £1.9 billion (+12%)
- Vaccines sales £2.7 billion (+2%); Shingrix £0.8 billion (+13%); Meningitis vaccines £0.5 billion (+5%); and Arexvy £0.3
billion (+36%) - General Medicines sales £2.5 billion (+4%); Trelegy £0.7 billion (+25%)
- Total operating profit >100% and Total EPS >100% driven by lower Significant legal expenses, lower CCL charges and
higher other operating income, partly offset by intangible asset impairments - Core operating profit +11% and Core EPS +14% reflecting Specialty Medicines and Vaccines growth, higher royalty income and disciplined increased investment in R&D portfolio progression in Oncology and Vaccines
- Cash generated from operations of £2.5 billion with free cash flow of £1.2 billion
| Q3 2025 | Year to date | |||||
|---|---|---|---|---|---|---|
| £m | % AER | % CER | £m | % AER | % CER | |
| Turnover | 8,547 | 7 | 8 | 24,049 | 3 | 6 |
| Total operating profit | 2,593 | >100 | >100 | 6,832 | >100 | >100 |
| Total operating margin % | 30.3% | 28.0ppts | 28.5ppts | 28.4% | 14.1ppts | 14.5ppts |
| Total EPS | 49.9p | >100 | >100 | 125.1p | >100 | >100 |
| Core operating profit | 2,985 | 8 | 11 | 8,149 | 6 | 9 |
| Core operating margin % | 34.9% | 0.4ppts | 0.9ppts | 33.9% | 0.7ppts | 1.0ppts |
| Core EPS | 55.0p | 11 | 14 | 146.3p | 7 | 11 |
| Cash generated from operations | 2,520 | 1 | 6.254 | 19 | ||
Pipeline progress and investment delivering future growth opportunities:
4 major new product approvals achieved so far this year:
- US & EU approvals for Blenrep for multiple myeloma, Penmenvy meningitis vaccine, Blujepa first-in-class antibiotic treatment for uUTIs and Nucala for COPD
- US decision on depemokimab (for asthma with type 2 inflammation, nasal polyps) expected in December 2025
15 scale opportunities with PYS potential >£2 billion now expected to launch 2025-2031:
- Pivotal trials started/to start by year-end for GSK'227 B7-H3 ADC for ES-SCLC; efimosfermin for treatment of MASH;
depemokimab for COPD; and GSK '981 (IDRx-42) for 2L GIST - Positive data support filings for tebipenem, potential new antibiotic for cUTIs; and Low Carbon Ventolin for asthma
Targeted business development further strengthens RI&I and Oncology pipeline:
- Agreement with Empirico Inc. to acquire first - and potentially best-in-class - oligonucleotide candidate to treat respiratory diseases
- Licensing agreement with Syndivia for early-stage ADC targeting prostate cancer
Continued commitment to shareholder returns
- Dividend declared of 16p for Q3 2025; 64p expected for full year 2025
- £1.1 billion spent in YTD 2025 as part of the £2 billion share buyback programme announced at FY 2024
2025 guidance upgraded
Now expect:
- 2025 turnover growth of between 6% to 7% (previously towards the top end of the range of between 3% to 5%);
- Core operating profit growth of between 9% to 11% (previously towards the top end of the range of between 6% to 8%); and
- Core EPS growth of between 10% to 12% (previously towards the top end of the range of between 6% to 8%)
Emma Walmsley, Chief Executive Officer, GSK:
“GSK’s momentum continues with another quarter of strong performance, supporting upgraded guidance for 2025, and positioning us well for 2026 and achieving our longer-term growth outlooks. Sales grew in all areas, with particularly strong performances in Specialty Medicines driven by double-digit growth in Respiratory Inflammation & Immunology, Oncology and HIV. We have also continued to make very good progress in R&D with four FDA product approvals so far this year, including for Blenrep in the US last week, and the start of pivotal trials and targeted business development to advance 15 scale pipeline opportunities, all launching before 2031. This is my final quarter reporting as CEO, and so I would like to thank everyone who has contributed to the transformation of GSK in the last nine years. Together, we have delivered a step-change in operating performance, new prospects for growth and a clear pathway for scale patient impact and sustained shareholder value. I am delighted to be passing the baton to Luke and to be leaving all that GSK has to offer in such good hands. I look forward to cheering him and everyone at GSK to further success.”
Assumptions and cautionary statement regarding forward-looking statements
The Group’s management believes that the assumptions outlined above are reasonable, and that the guidance,
outlooks, and expectations described in this report are achievable based on those assumptions. However, given the
forward-looking nature of these guidance, outlooks, and expectations, they are subject to greater uncertainty, including
potential material impacts if the above assumptions are not realised, and other material impacts related to foreign
exchange fluctuations, macro-economic activity, the impact of outbreaks, epidemics or pandemics, changes in
legislation, regulation, government actions, including the impact of any potential tariffs or other restrictive trade policies
on the Group's products, or intellectual property protection, product development and approvals, actions by our
competitors, and other risks inherent to the industries in which we operate.
This document contains statements that are, or may be deemed to be, “forward-looking statements”. Forward-looking
statements give the Group’s current expectations or forecasts of future events. An investor can identify these
statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’,
‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in
connection with any discussion of future operating or financial performance. In particular, these include statements
relating to future actions, prospective products or product approvals, future performance or results of current and
anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, dividend
payments and financial results. Other than in accordance with its legal or regulatory obligations (including under the
Market Abuse Regulation, the UK Listing Rules and the Disclosure Guidance and Transparency Rules of the Financial
Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result
of new information, future events or otherwise. The reader should, however, consult any additional disclosures that the
Group may make in any documents which it publishes and/or files with the SEC. All readers, wherever located, should
take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and
investors are cautioned not to place undue reliance on the forward-looking statements.
All guidance, outlooks and expectations should be read together with the guidance and outlooks, assumptions and
cautionary statements in this Q3 2025 earnings release and in the Group's 2024 Annual Report on Form 20-F.
Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to
factors that are beyond the Group’s control or precise estimate. The Group cautions investors that a number of
important factors, including those in this document, could cause actual results to differ materially from those expressed
or implied in any forward-looking statement. Such factors include, but are not limited to, those discussed under ‘Risk
Factors’ in the Group’s Annual Report on Form 20-F for 2024. Any forward-looking statements made by or on behalf of
the Group speak only as of the date they are made and are based upon the knowledge and information available to
the Directors on the date of this report.



