GSK delivers improvements in sales, margins and cash flow in 2017
Issued: London UK
Total EPS 31.4p, +67% AER, +36% CER; Adjusted EPS 111.8p, +11% AER, +4% CER
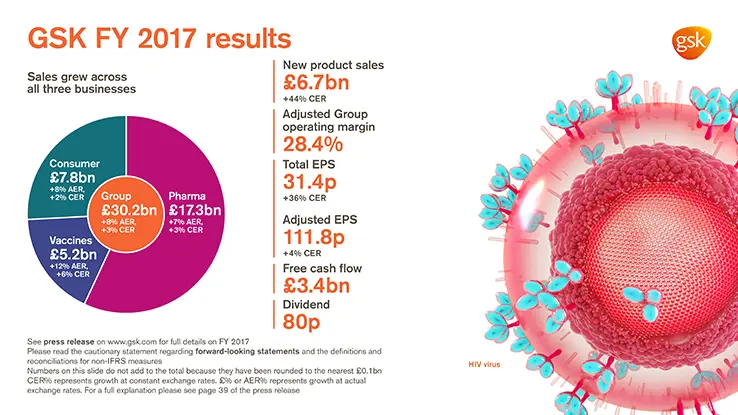
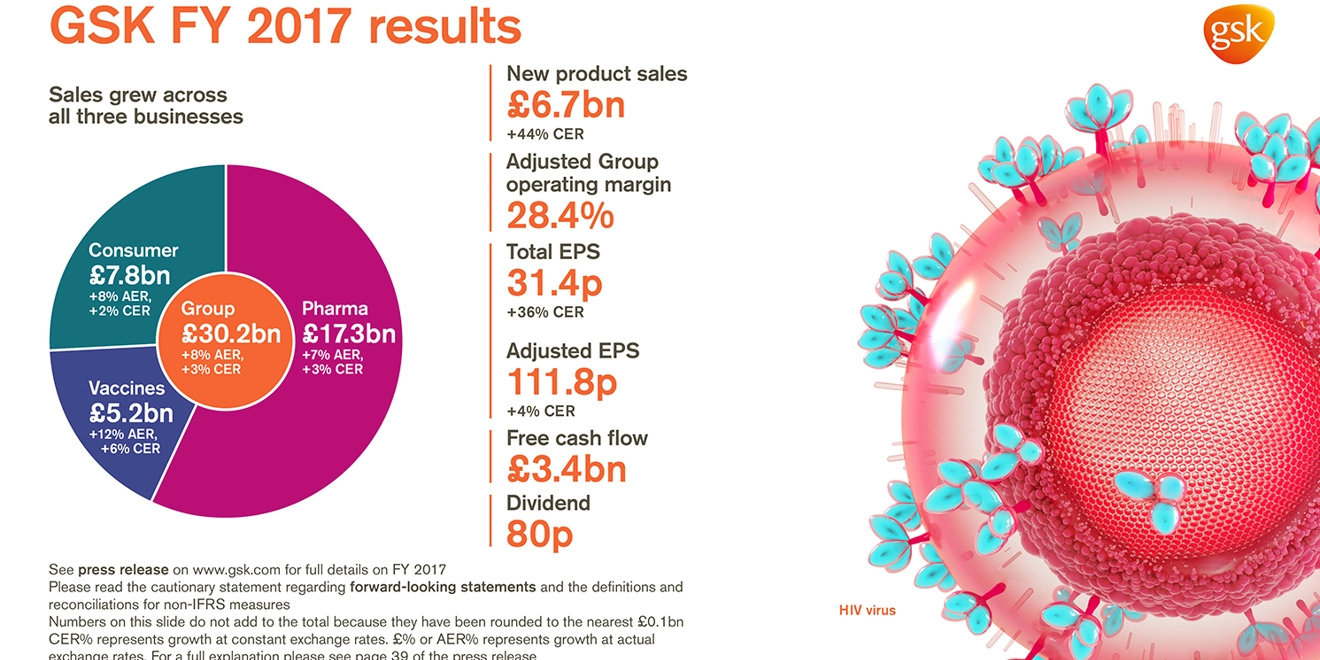
2017 Financial highlights
- Turnover £30.2 billion, +8% AER, +3% CER
- Sales growth across all 3 businesses: Pharmaceuticals £17.3 billion, +7% AER, +3%, CER;
Vaccines £5.2 billion, +12% AER, +6% CER; Consumer Healthcare £7.8 billion, +8% AER, +2% CER - Improved Adjusted Group operating margin of 28.4% (2016: 27.5%). Pharmaceuticals 34.3%;
Vaccines 31.9%; Consumer Healthcare 17.7% - Total EPS 31.4p, after accounting charges of £1.6 billion related to US tax reform
- Adjusted EPS 111.8p, +11% AER, +4% CER, in line with 2017 guidance
- 2017 free cash flow of £3.4 billion (2016: £3.0 billion)
- 23p dividend declared for quarter; 80p for 2017
2018 financial guidance
- 2018 Adjusted EPS Guidance: Growth is subject to uncertainty of timing and impact of possible generic competition to Advair in the US:
In the event of no substitutable generic competitor to Advair in the US, expect 2018 Adjusted EPS growth to be 4 to 7% CER
In the event of a mid-year introduction of a substitutable generic competitor to Advair in the US, expect full year 2018 US Advair sales of around £750 million at CER (US$1.30/£1) with Adjusted EPS flat to down 3% CER
Both scenarios reflect the benefit of US tax reform with expected 2018 effective tax rate on Adjusted profits of 19-20% - Continue to expect 80p dividend for 2018
Product and pipeline highlights
- New product sales of £6.7 billion, +51% AER, +44% CER, driven by strong performances from Tivicay and Triumeq in HIV, the inhaled Ellipta portfolio and Nucala in Respiratory and meningitis vaccines
- Three key approvals: Shingrix vaccine for shingles; Trelegy Ellipta, once-daily single inhaler triple therapy for COPD; Juluca (dolutegravir and rilpivirine), first 2-drug regimen, once-daily, single pill for HIV
- Preferential recommendation for Shingrix received from US CDC
- Trelegy Ellipta approved in Europe for COPD
- Nucala filed in US for eosinophilic COPD
- Phase III HIV treatment study initiated investigating long-acting 2-drug regimen of cabotegravir plus rilpivirine administered every two months
- In Oncology, Breakthrough Therapy Designation received from FDA for BCMA antibody-drug conjugate for relapsed and refractory multiple myeloma. Positive BCMA data presented at ASH meeting
Emma Walmsley, Chief Executive Officer, GSK said:
“In 2017 GSK delivered encouraging results from across the company with sales growth in each of our three global businesses, an improved Group operating margin, Adjusted EPS growth of 4% (CER) and stronger free cash flow.
“We are focused on competing effectively across our current portfolio and delivering three new launches which bring significant benefits to patients: Trelegy Ellipta which provides three medicines in a single inhaler to treat COPD; Juluca, the first 2-drug regimen, once-daily, single pill for HIV, helping to reduce the amount of medicines needed, and Shingrix, our new vaccine which represents a new standard for the prevention of shingles.
“Improving our Pharmaceuticals business remains our main priority and we are strengthening our pipeline with a focus on priority assets in two current therapy areas, Respiratory and HIV, and two potential areas, Oncology and Immuno-inflammation. We will provide a further update to investors at Q2 on our plans for R&D.
“We continue to make changes across GSK to drive improvements in performance and we have made several new appointments to key leadership positions.
“Looking ahead, in 2018 we could see a potential generic version of Advair in the US and our 2018 guidance reflects this. With the sales momentum we anticipate from new and recent launches and focused improvements in operating performance we are increasingly confident in our ability to deliver mid to high single digit growth in Adjusted EPS CAGR (2016-2020 at 2015 CER).
“Cash generation also continues to be a key focus with free cash flow for the year improving to £3.4 billion. We met our commitment to pay a total dividend of 80p for 2017 and continue to expect to pay 80p for 2018.
“Finally, I would like to thank all our customers, suppliers and employees for their support and hard work in 2017 and look forward to working with them in 2018 and beyond to deliver our strategic priorities and improved performance for GSK.”

Watch Emma Walmsley, CEO, summarise our performance in 2017

Watch Simon Dingemans, CFO, give an overview of our financial results for 2017

Watch Luke Miels, President, Global Pharmaceuticals, set out his priorities for our pharmaceuticals business
Broadcast quality versions of these films are available on request for use by media from our Video footage library. To request access, please contact us through our media relations team on +44 (0)20 8047 5502 or email corporate.media@gsk.com
About GSK
GSK – one of the world’s leading research-based pharmaceutical and healthcare companies – is committed to improving the quality of human life by enabling people to do more, feel better and live longer. For further information please visit www.gsk.com/about-us.
Cautionary statement regarding forward-looking statements
This document contains statements that are, or may be deemed to be, “forward-looking statements”. Forward-looking statements give the Group’s current expectations or forecasts of future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results. Other than in accordance with its legal or regulatory obligations (including under the Market Abuse Regulation, the UK Listing Rules and the Disclosure and Transparency Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. The reader should, however, consult any additional disclosures that the Group may make in any documents which it publishes and/or files with the SEC. All readers, wherever located, should take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned not to place undue reliance on the forward-looking statements.
Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the Group’s control or precise estimate. The Group cautions investors that a number of important factors, including those in this document, could cause actual results to differ materially from those expressed or implied in any forward-looking statement. Such factors include, but are not limited to, those discussed under Item 3.D ‘Risk Factors’ in the Group’s Annual Report on Form 20-F for 2016. Any forward looking statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information available to the Directors on the date of this report.